2025 marked a clear turning point for laboratory technology. The year moved the industry beyond incremental digital upgrades and into a phase where intelligence, automation, and compliance-driven design became central to everyday lab operations. For CrelioHealth, this transformation was not about chasing trends but about delivering practical, high‑impact innovation that helped laboratories work smarter, stay compliant, and scale with confidence.
The year 2025 was defined by deeper product intelligence, stronger regulatory alignment, and closer collaboration with customers and partners worldwide. Below is a closer look at how the year unfolded.
1. Making Labs Smarter & More Intelligent for the Future
One of the strongest themes of 2025 for CrelioHealth was intelligence at scale. Leveraging the power of artificial intelligence and automation, we focused on eliminating uncertain and inessential manual efforts that consumed time and contributed to inefficiencies. Smart innovations broadly helped labs eliminate operational blind spots and oversight over complex areas, eased manageability, and helped labs act on data faster and with greater confidence.
AI‑Driven Capabilities That Reduce Manual Work
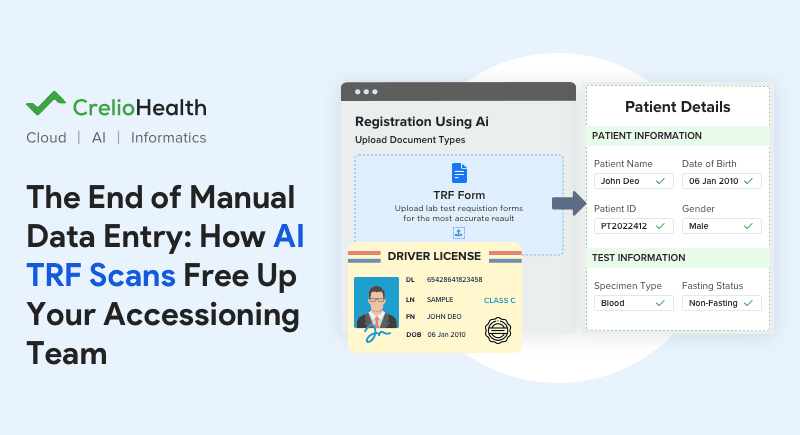
The introduction of AI in Crelio Systems helped customers manage high-volume and document-heavy workflows in 2025. A major highlight was the introduction of Bulk Upload for AI-TRF Scan, which enabled them to process large numbers of test requisition forms quickly and accurately. By automating data extraction and validation, labs were able to reduce data entry errors, shorten accessioning times, and free teams to focus on higher‑value tasks.
AI summaries in analytical dashboards helped labs effortlessly compare data and get better outcomes from complex, laborious analysis. AI-assisted descriptions saved pathologists from typing repetitive specimen and result descriptions. These improvements weren’t about replacing human expertise, but about supporting it, allowing teams to focus their attention where it matters most.
A few AI-centered LIMS updates include:
Purpose‑Built Workflows for Complex Lab Modalities
As laboratories expanded into specialized and high-complexity testing, the need for dedicated, modality-specific workflows became increasingly apparent. In 2025, CrelioHealth introduced new automated workflows designed to handle complex lab processes without forcing teams into rigid systems.
These workflows helped labs standardize processes, reduce dependency on manual interventions, and maintain consistency across departments, even as test volumes and service offerings grew.
Top Features To Look Out For:
Smarter Dashboards and End‑to‑End Tracking
Operational visibility remained a key priority throughout the year. Several new dashboards and tracking capabilities were introduced to help labs monitor critical activities in real time:
- Sample Vial Tracking improved traceability across the sample lifecycle, reducing losses and strengthening audit readiness.
- Auto‑Reruns and Sample Rerun Triggers ensured critical parameters were automatically reprocessed when required, improving result accuracy and turnaround times.
- Claims and Remittance Tracking Dashboards gave labs clearer insight into billing performance, outstanding claims, and reconciliation workflows.
Together, these enhancements helped laboratories move from reactive problem‑solving to proactive operational control.
In-Built Smart Lab Reports with Ready-to-Use Templates
Reporting remains one of the most critical touchpoints between labs and clinicians. In 2025, CrelioHealth strengthened this layer with Smart Lab Reports powered by pre-available, customizable templates.
These enhancements helped laboratories:
- Generate consistent, professional reports faster
- Reduce approval cycles through bulk parameter validation
- Maintain compliance while adapting report formats to clinician needs
By simplifying report creation and approvals, labs could focus more on quality and less on repetitive administrative work.
2. Prioritising Technology Evolution & Compliance
Healthcare innovation is only meaningful when it aligns with regulatory expectations. In 2025, compliance and technology evolution went hand in hand across our platform.
![]()
Supporting National‑Scale Healthcare Initiatives
A key milestone this year was the successful onboarding of 14 Ministry of Health (MOH) regional laboratories in the Kingdom of Saudi Arabia (KSA). This initiative demonstrated CrelioHealth’s ability to support large‑scale, government‑driven healthcare programs while meeting regional regulatory and operational requirements.
Our platform enabled these labs to modernize their SOPs, standardize workflows, and ensure consistent reporting across regions, without disrupting existing operations.
Helping Labs Stay Ahead of Regulatory Requirements
Throughout 2025, continuous product updates enabled laboratories to align with evolving regulatory standards, including CLIA, CAP, CCPA, ISO, and other state and country‑specific mandates. Rather than treating compliance as a one‑time requirement, our approach focused on embedding regulatory readiness directly into workflows.
From audit‑ready documentation to controlled access and traceable actions, the system supported labs in maintaining compliance while improving operational efficiency.
Year‑on‑Year Commitment to Data Security & Privacy
Data protection remained a foundational priority. Our systems, processes, and data communication practices continued to align with globally recognised standards such as HIPAA, SOC 2, GDPR, and ISO. Regular updates and reviews ensured that security controls evolved alongside emerging risks, helping customers maintain trust with patients, partners, and regulators year after year.
3. Strengthening Connections with Clients & Partners
![]()
2025 was also about listening, learning, and engaging more closely with the global laboratory community.
Industry Events and Global Presence
CrelioHealth maintained an active presence across ADLM events in the USA, IBMS Congress, UK, and Medlab, Middle East, connecting with laboratory professionals, thought leaders, and technology partners. These interactions offered valuable insights into real‑world lab challenges and helped shape future product direction.
Event highlights, on‑ground interactions, and video glimpses captured the energy and collaboration that defined these engagements.
Knowledge‑Sharing Webinars, Strategic Sponsorships, and Partnerships
CrelioHealth actively participated in the Executive War College, 2025, as the lunch sponsor and partner. Connecting with industry leaders, we successfully illustrated that our cloud infrastructure, AI, and data-driven tools are streamlining lab operations at scale.
Such webinars continued to be a strong channel for sharing expertise and practical guidance. Topics ranged from operational efficiency and compliance to automation and digital transformation, helping labs navigate change with clarity and confidence.
4. Acknowledgement: Stepping into a New Year with Renewed Focus
As 2025 came to a close, we extend our sincere appreciation to our customers, partners, and collaborators. Your trust, feedback, and continued support have played a critical role in shaping every milestone achieved this year.
The year reinforced a simple belief: sustainable lab transformation happens when technology adapts to real workflows, regulatory realities, and human expertise. As we move into the next phase, our focus remains on building smarter, more connected, and future‑ready laboratory solutions.
With new goals on the horizon and a clear vision ahead, we look forward to continuing this journey, together, with the global laboratory community.